Platelet-derived Integrin and Tetraspanin-enriched Tether (PITT)
Platelets are mediators of haemostasis and thrombosis, but also increasingly recognized as versatile effector cells of innate immunity. The concept of thrombo-inflammation recognizes that thrombotic and inflammatory pathomechanisms are closely intertwined.
Platelets thereby act as central orchestrators of immune cell trafficking, vascular barrier function and organ integrity and contribute to organ injury in severe disorders with limited treatment options (e.g. stroke, sepsis, ARDS). The underlying molecular mechanisms are not known.
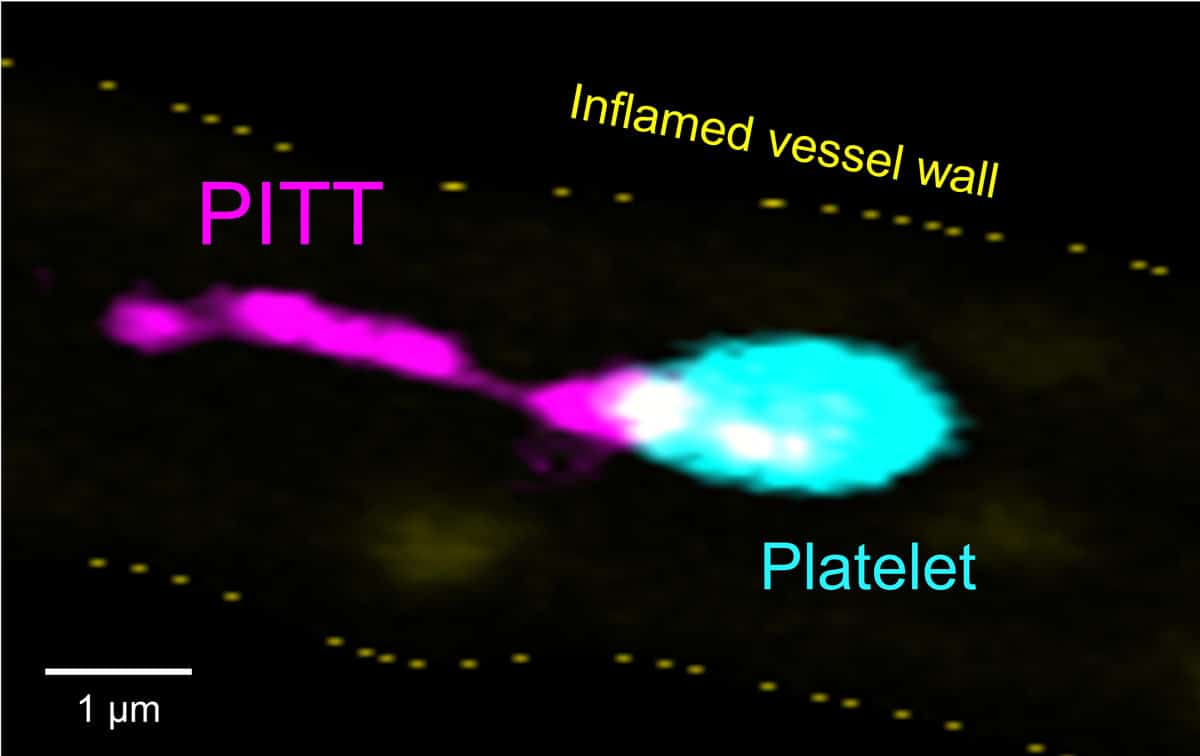
Bernhard Nieswandt and his team recently discovered an unexpected novel effector mechanism in platelets with potentially huge impact for understanding these cells in health and disease: Resting platelets can rapidly reorganize the entire pool of their principal adhesion receptor, integrin αIIbβ3, along with its associated tetraspanins and signalling machinery into ´disintegration´ complexes (DISCs) in distinct membrane microdomains.
These DISCs serve as building blocks for a novel organelle, the ´Platelet-derived Integrin and Tetraspanin-enriched Tether´ (PITT). PITTs are loaded with signalling molecules, ribosomes and RNA and can segregate from the platelet to promote thrombo-inflammation.
Also β1-integrins as well as glycoprotein (GP)VI can be integrated into DISCs and deposited at discrete adhesion points.
We postulate that circulating platelets have the capacity to use their principal adhesion/signalling machineries in two fundamentally different ways and thereby switch between the haemostatic and a thrombo-inflammatory effector programme. If proven correct, this would implicate a radically new paradigm in platelet biology, and open new avenues for the treatment of a wide range of diseases with major societal impact.
PITT-Inflame will
- provide a detailed molecular composition and architecture of DISCs and PITTs
- decipher underlying signalling networks
- identify PITT-induced effects on target cells
- deduce therapeutic strategies


